CRYSTALLOGRAPHY
Crystallography
is the science that studies the geometry of crystals. That is the shape and
forms that minerals assume in space in 3 dimensions (the regular patterns and interfacial
angles of crystals).
Word History:
The
word crystal,was derived from a Greek
word krustallos meaning ‘clear ice’ formed by the freezing of
water. The ancient Greeks were amazed by
quartz (a mineral and rock) which occurred in forms having a characteristic
shape and being bounded by flat surfaces (faces). From the transparency of quartz, together
with the presence of inclusions in it, it was thought that quartz resulted from
the freezing of water under intense cold and the name krustallos was given to it. The application of this name was later
extended to all minerals that showed forms with smooth surfaces. These forms are crystals and their study is
crystallography.
Definition:
A
crystal can be defined as a homogenous solid bounded by naturally formed plane
(or smooth) surfaces called faces which can be related to a regular internal
arrangement of atoms.
Crystals
are formed by a process called crystallization during the solidification of
minerals from the gaseous or liquid states or from solutions of magmatic origin
It
is the regular internal arrangement of atoms within a mineral which really
defines whether or not a mineral is crystalline. The study of the outward appearance of
minerals (crystals) is important to geologists because it helps them identify
and recognize different minerals. This
is possible because different minerals have different chemical compositions and
different internal atomic arrangements and therefore they have different
crystal shapes and forms.
The
Unit Cell
The
structure of every crystal is a construction of atoms or groups of atoms
arranged in three dimensional patterns which are repeated throughout the
crystal.
A unit cell is
the smallest complete unit of pattern of a crystal. Many unit cells combine in repetition to form
a whole crystalfor example;
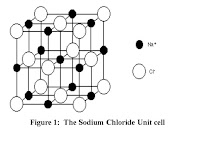
Sodium Chloride
(rock salt), a cubic mineral is an excellent example of a mineral with a cubic
unit cell. Here, the unit cell consists
of a cube with sodium ions at the corners and at the center of the faces and
chlorine ions at the center of the edges of the cube as shown below:
The
unit cell in sodium chloride can be interpreted as having 8 small cubes with
either sodium or chlorine ions at the corners.
There
are 7 major types of unit cells namely:
-
The Cubic unit cell
-
The Tetragonal unit cell
-
The Hexagonal unit cell
-
The Trigonal unit cell
-
The Orthorhombic unit cell
-
The Monoclinic unit cell
-
The Triclinic unit cell
They
give rise to seven systems under which crystals can be classified. Some of the unit cells are further subdivided
to give 14 in all. These give the 14
types of space lattice that can be constructed from them sometimes called the [1]Bravais
Lattices.
[1]Bravais lattices are named after Auguste
Bravais, a French physicist in the early 18th century who worked on
crystal structure.
Figure 2: The 14 types of
space lattice (Bravais Lattices)
The
external morphology of a crystal (like faces, edges, angles and form) and the
general symmetry of any crystal are determined by the internal arrangement of
these unit cells since they are the building block for crystals.
Crystals can be
formed by solidification from liquid or gaseous states or by precipitation from
solutions saturated with ions. All these
processes are called crystallization.
Crystallisation
Crystallization
is a process by which crystals are formed.
Naturally occurring crystals (mineral crystals) are usually formed from
a solution or a melt. In a liquid state
or a solution, the atoms and ions are distributed haphazardly, but when the
temperatures and pressure of the solution begin to drop, they quickly arrange
themselves in an orderly manner forming crystalline solids called crystals.
A
good example of crystallization can be seen during the formation of minerals
from magma (molten matter). In the
molten state, the magma contains ions which are randomly dispersed within the
melt. As the temperature and pressure
begin to drop, various ions are attracted to each other forming crystals of
different minerals. If the process of
cooling is slow and gradual, the ions will have enough time to migrate and come
together thereby building large but few and well-formed crystals with smooth
faces. However, if the drop in
temperature is rapid, no time is allowed for the ions to move and coalesce
(come together) so several centers or units of crystallization are developed
around which many but irregularly oriented crystals are formed. Such crystals lack flat surfaces.
The
degree of crystallization affects the development of crystals which is
reflected on the external form and shape of the crystal.
Based
on the degree of crystalinity and the development of external form of crystals,
the following terms can be used to describe crystals:
i.
Euhedral:It
is a term that describes crystals that are well formed with smooth faces. Very few minerals show good forms. This indicates that they are formed under
suitable conditions and such minerals are said to be crystallized.
ii.
Subhedral:It
is a term that describes crystals with partially formed or imperfectly formed
faces. They are said to be crystalline.
iii.
Anhedral:It
is a term that that describes minerals that completely lack crystal faces.
According
to the fineness of the grains of crystals, the following terms are used to
describe minerals:
i.
Microcrystalline:Describes
minerals with fine grain aggregates, which can only be studied using
microscopes.
ii.
Cryptocrystalline:Describes
minerals with crystal aggregates that are so fine grained that the individual
grains cannot be seen using the microscope but can only be detected by the use
of X-ray diffraction techniques. For example Chalcedony.
iii.
Amorphous: These
are minerals which lack any ordered internal arrangement of atoms for example
Opal

No comments:
Post a Comment